What is so important about nitrogen?
 In the world of human economy, politics, and society, there are many different currencies, all of which are traded for goods. This could be oil, US dollars, Facebook likes or Grandma’s cookies. The natural world has similar currencies—trading in oxygen, carbon, and perhaps the most precious currency of all: nitrogen.
In the world of human economy, politics, and society, there are many different currencies, all of which are traded for goods. This could be oil, US dollars, Facebook likes or Grandma’s cookies. The natural world has similar currencies—trading in oxygen, carbon, and perhaps the most precious currency of all: nitrogen.
The whole world fights for it, harvesting it honestly, cheating for it, and stealing it brutally. It is a powerful element, required in the highest quantities for good growth, defense, and reproduction. This article will cover what nitrogen is, where it occurs in the system, and how it interacts with the other elements of aquaponics. We will also discuss how to identify a nitrogen deficiency.
Nitrogen in aquaponics
As I have already mentioned, nitrogen is one of the most important nutrients in your aquaponic system. It occurs in several forms in the natural world, but for now, we will focus on the most relevant: protein.
Protein is built from amino acids, which are themselves built from nitrogen. All plants and animals contain proteins, and when they die, other organisms consume them and scavenge these proteins for energy. Thus, nitrogen enters the body in the form of proteinous food.
The nitrogen cycle
When land animals consume proteins they eventually break them down into amino acids and then to ammonia. Ammonia (NH3) is nasty stuff—it’s very toxic—and the best way to get rid of excess ammonia is to excrete it. So, in land animals, ammonia is converted to a chemical called urea and excreted in urine.
Nitrogen occurs in aquaponic systems in much the same way. It enters in the form of fish feed. The fish consume the food but the process is simpler; the microbes in their bellies break the proteins down to ammonia and ammonium. Ammonia is usually present as ammonium (NH4+) which moves across the cell membranes of the fish and eventually diffuses out into the water. No extra conversion necessary. For fish, anyway.
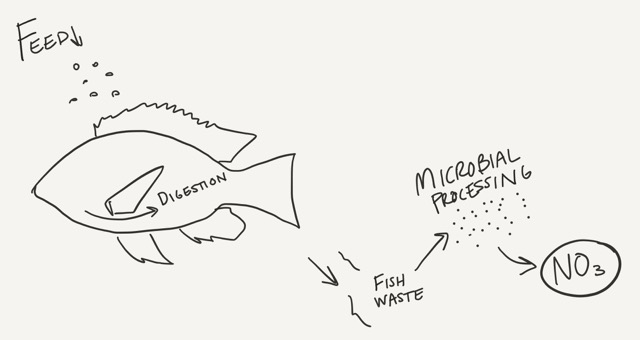
Depending on the pH of your water, ammonium may stay ammonium or turn into ammonia, which can be very dangerous. Ammonia has no charge, so the fish have difficulty keeping it out of their bodies. When this happens, the fish are poisoned.
So, once ammonia is in the solution, it must be transformed or it will eventually kill the fish. There are two ways to do this: alter your pH to favor ammonium (which is not suggested) or convert the ammonia into nitrates. The series of transformations from an organic form (ammonia) to a plant-available form (nitrate)—and the next step in the cycle—is called nitrification.
Nitrification
Nitrification is the process that drives most aquaponic systems. Essentially, nitrification converts ammonia and ammonium into useful nitrate. This happens in two processes: turning ammonia into nitrite, and turning nitrite into nitrate.
In almost all environments (except anaerobic environments) ammonia is quickly transformed into nitrite (NO2-). Microbes—or nitrifying bacteria—in the soil or solution add oxygen to (or oxidize) the ammonia. While this is happening, the microbes get the energy to fix carbon (break carbon off of carbon dioxide to build cells). In addition, hydrogen ions (H+) are produced—the very ions that are measured in the pH test and cause water to become acidic.
This process has traditionally been attributed to a bacterium called Nitrosomonas. Recent research shows that there are many hundreds, if not thousands of different species in addition to Nitrosomonas that also do this work.
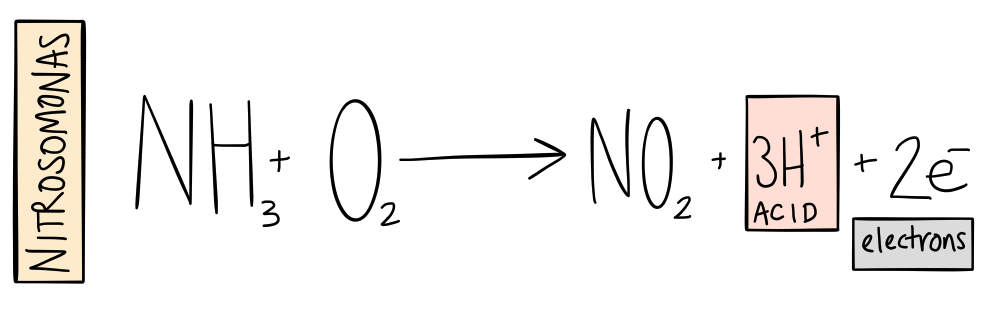
That is step one.
The next step in the cycle is to convert that nitrite into nitrate. Nitrite is also quite toxic so you never want too much in your system. Fortunately, it represents a lot of stored energy to other [nitrifying] bacteria. These bacteria oxidize the nitrite and use the energy from the process to fix more carbon. Sounds familiar, except this time the result is nitrate (NO3-). Nitrate is a relatively non-toxic form of nitrogen that plants can take up and use to build cells.
The bacterium that has been most commonly recognized for performing this chemical reaction is called Nitrobacter. Again, however, research indicates that there are many bacteria that participate in this reaction besides Nitrobacter.
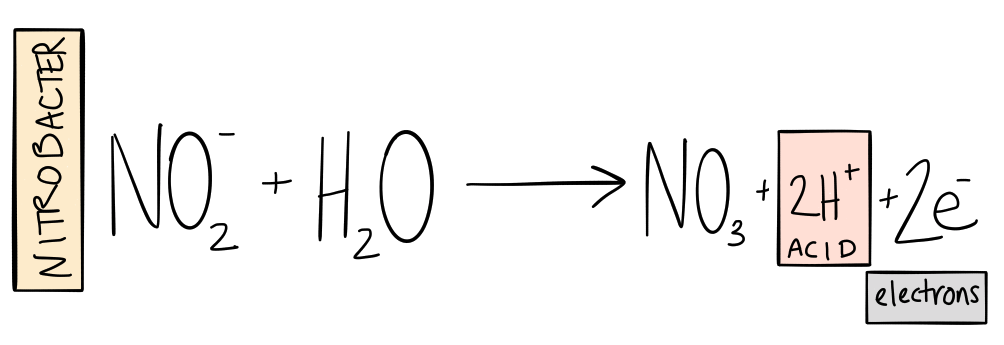
As the bacteria oxidize ammonia and nitrite, they release hydronium ions into the solution, making the system more acidic. (For people who want to run their systems in the optimal pH range for nutrient availability, nitrification is the single most important process for lowering pH). This indicates why the solution of older systems trends toward more acidic pH values.
Nitrogen is available at a broad number of pH values—so pH matters less when it comes to nitrogen availability.
“…nitrification is the single most important process for lowering pH.”
Nitrification efficiency and pH
The speed at which the pH of a solution changes, however, can influence the form of nitrogen available (see the video on ammonia/ammonium and pH) as well as the efficiency of nitrification. If nitrifying bacteria are not given time to adjust to changing pH levels (like almost any other system variable), nitrification will suffer.
In fact, nitrification proceeds just fine at low pH values so long as the nitrifying ecology is given time to adjust. Nitrifying bacteria are generally inefficient when it comes to changing system variables. They often die off or go dormant when exposed to too much light, temperature fluctuations, fluctuations in salinity and pH, as well as many other changes to their environment.
Nitrifying bacteria in aquaponics
The perceived balance between pH and nitrification efficiency has been based on the assumption that nitrification activity in aquaponic systems was primarily a function of two different groups of bacteria: Nitrosomonas spp., and Nitrobacter spp.
In lab trials, these bacterial species have shown sensitivity to pH, with changes in pH affecting their ability to oxidize ammonia (Nitrosomonas) and nitrite (Nitrobacter). Remember that most nitrifying bacteria (studied so far at least) don’t handle changing environmental variables well. This is important to know for two reasons:
- Changing your pH rapidly will reduce your nitrification efficiency.
- Most nitrifiers are very hard to remove from the environment and culture in a lab.
Why do I need to know this?
What does this have to do with the nitrification debate? Well, point #1 tells us that perhaps many of the “system crashes” ascribed to running a system pH too low may actually be attributed to decreasing system pH too quickly. Point #2 tells us that Nitrosomonas and Nitrobacter may not really be the most important nitrifiers in systems—they’re simply the easiest groups to isolate and grow in a petri dish in a lab.
What does this mean?
It basically means that the hard and fast rules of nitrification may not be as hard and fast as they’re typically communicated. There are many systems out there running very low pH ranges with great nitrification efficiency (including ours). It may be that Nitrosomonas and Nitrobacter species are the primary nitrifiers in our system, but the reality is that we just don’t know. What we do know is that our nitrification is efficient and excellent regardless of our system pH.
Nitrification in soil
To put this into perspective, there are many acidic soils and marine environments around the world where nitrification occurs at remarkably low pH ranges. Many of the nitrifiers in these environments aren’t members of the Nitrobacter or Nitrosomonas groups. Many of them are unknown. In a single shovel of soil, there’s an estimated 10,000 different species of bacteria or roughly twice the number of bacteria currently known to science.
With that in mind, I think that it’s not just possible, but probable that there are some pretty interesting bacteria performing nitrifying functions in aquaponic systems around the world.
Check out our BSA video detailing Biological Surface Area—arguably the most important system design element that fosters nitrification.
Nitrate
 In any case, the end product is nitrate (NO3-). Some plants can take up ammonium and use it. However, most prefer nitrate. In systems where there is an excess of ammonium, the plants can tend to be leggier and are often less salable. On the other hand, in systems with lots of nitrates, the problems with aphids and other pests can be more dramatic, requiring more intervention. So be aware that systems with too many nitrates can see increased issues with pests.
In any case, the end product is nitrate (NO3-). Some plants can take up ammonium and use it. However, most prefer nitrate. In systems where there is an excess of ammonium, the plants can tend to be leggier and are often less salable. On the other hand, in systems with lots of nitrates, the problems with aphids and other pests can be more dramatic, requiring more intervention. So be aware that systems with too many nitrates can see increased issues with pests.
Nitrate, nitrite, and ammonia levels can be tested easily with a freshwater test kit, like this one. Nitrate dissolves in the solution and is instantaneously competed for by bacteria, fungi, algae and other plants. All of these organisms are taking nitrate up and using it in their tissues. As the bacteria, fungi, and algae die, that nitrogen (often in the form of protein) re-enters the system and the cycle begins again. Much of the nitrate, however, is delivered, safe and sound, to the root zone, where the plants in your system take it up and use it to grow.
Ideal nitrate levels
While it is dangerous to have ammonia or nitrite levels much above 2 ppm and 1 ppm respectively, nitrate can often run well above 100 ppm (well off the chart for many nitrate tests) without posing a threat to your fish. Many hydroponic systems run nitrate in the range of 160 ppm. Plants can often appreciate levels even higher than that, but the aquaponic grower must strike a balance between the needs of the fish, the system ecology (including pests), and the needs of the plants. For this reason, I recommend that most aquaponic growers shoot to maintain their nitrate in the range of 40-80 ppm for good, consistent plant growth.
Maintaining consistent nitrate ranges
Many systems have a hard time maintaining nitrogen levels, especially as the system matures, plants get large and the system ecology becomes more complex. This may require feeding to be increased to meet the increased demand. Many folks initially want to increase stocking density, but this is often a mistake. Instead, increase feeding rates (but don’t overfeed!), and see if higher nitrate levels can be achieved with the same amount of fish.
How to identify a nitrogen deficiency
Since nitrogen is a mobile nutrient (it is directed to different places within the plant), deficiencies affect older growth first. The symptoms of a deficiency are total chlorosis without a pattern on the leaf and stunted growth. Read our Beginner’s Guide to Nutrient Deficiencies for more.
N-P-K
In addition to fish feed, nitrogen enters the system through fertilizers. Almost all fertilizers have an NPK rating telling you the relative concentrations of nitrogen, phosphorus, and potassium (in that order). For vegetative growth (the growth of stems, leaves, and roots), nitrogen is required more than any other mineral nutrient.
Conclusion
In many ways, nitrogen is the most important plant nutrient, but also the simplest. The next nutrient on our list is iron—a finicky nutrient but an important one.




Hi Dr. Nate Storey,
I really enjoyed your explanations about the nutrients deficiency and solutions. I have 1 year old backward Aquaponic system . More than 100 tilapias and 4000 liters system including fish tank,DWC and growbeds.
Somehow, I am struggling in increasing the nitrate level. My system always shows less than 5 ppm nitrate. I have a big ridge gourd plant and 20-spinach plant. PH is around 6.9.Any suggestion to boost the nitrate, already increased the maximum feeding rate (200 gm) 3 times a day.
Thanks,
Ankur
Hi Ankur,
Do your plants look good? If they do and they’re growing well, then you’re probably not measuring higher nitrate ppm because the plants are consuming it.
If they do not look well, you could potentially change the feed to have a higher protein content. Also, depending on how big the gourd is (if it is fruiting), that could be taking up a bunch of nitrates. Try taking that out and grow many smaller plants to replace it.
Hope this helps!
I just treated my calcium with a product called *growth technology, liquid cal/mag/nit
It raised my nitrate by a shit load.
5ppm up to 80ppm
While my calcium only went from 50ppm to 100ppm
I used 200mls of the mixture instead of the recommened 3.5 litres.
I wont be using this product again but it may help you out.
At the very end you touched important issue but without explanation. Could you please either in the article or in the comment reply explain how can we convert known nitrogen ppm in the aquaponics water to N (NPK) number typically used to measure fertilizer NPK numbers? Thanks!
Hi Madus,
The numbers on a fertilizer bag representing NPK are ratios. The nutrients that you test in ppm in your system after dosing should match that ratio—in other words, the ppms of the nutrients in your system should be in the same proportion as the numbers on the bag.
Hi I am looking into starting a simple aquaponics system at home. I was curious if I need to.pre treat the water that my fish will be in with nitrates or not. And if so how do I do that
Hey Samantha, I have just started a small aquaponic system myself.
Although i have not much experience in aquaponics, i have been keeping fish and aquariums for some time now.
The basic requirement is to setup your tank and biological filter/growbed.
Then feed the tank slightly so as to generate ammonia to start off the nitrogen cycle.
Feed small amount per day, remove if food goes mouldy. Test water parameters every other day. You should see a rise in ammonia first. Then a quick decline to 0ppm
After this you should see a rise in Nitrite, this may take some time to drop back down but eventually will drop to 0ppm.
At this point, you should be reading some Nitrates, anywhere from 10ppm-40 is a good start.
Your tank is now ready for fish.
Add fish slowly so as to not overwhelm the beneficial bacteria that is converting the ammonia>nitrite>nitrate. Check levels every other day. When you know your fish are happy and healthy, you can start to plant some plants in your grow bed 🙂
Please dont take this as gospel as i am still new to aquaponics, but i hope this gives you a bit of insight into the nitrogen cycle and helps you take the plunge
Very informative article explains the basic working of an Aquaponics system, please suggest the ideal ratio of Fish tank to Grow bed to Plants.
Hello, My nitrates readings at 10 mg/l. using the highest fishfeed protein levels. I was wondering if I can add a very low rate of NPK chemical solution to the system to increase the nitrate level for better growth ? thanks
Hi,
I really enjoyed reading your content on aquaponics. I am willing to start my own aquaponics system on terrace. Your article made it clear on pH values and other factors influencing the growth of the plants. Will like to more about fish breeding systems in aquaponics tank system.
Thanks!
Aditya Abhishek
Thanks for the information you have provided.
I’m new to this concept.
1) need to know in which part of the system ammonia was converted into nitrites and nitrates.
2)Things used to makes these ammonia into plant nutrient nitrate
CAN you please explain in detail.