Warning! This post gets a little technical. To get straight to supplementing info, skip to “How to supplement iron in aquaponics” near the bottom of the post.
Why is iron important?
In mass, iron is one of the most plentiful elements on the planet and one of the oldest metals known to and used by humanity. It is also an important plant and animal nutrient and thus crucial to your aquaponics system.
Iron deficiency typically shows as yellowing only between the veins, with the veins remaining green. Young growth is most affected.
In animals, the most common iron-containing substance is heme complexes. We’re most familiar with hemoglobin. In hemoglobin, iron helps bind oxygen for transport throughout the body.
In plants, iron serves many functions but is an essential component in the production of chlorophyll, the site of photosynthesis.
Without enough iron, plants cannot produce enough chlorophyll, leading to retarded plant growth characterized by interveinal chlorosis. Iron is also a key component of cytochrome—a hemeprotein that plays a key role in ATP generation—the currency of cellular metabolism.
In this capacity, it is irreplaceable to both plants and animals. Iron also plays a major role in many other proteins and reactions.
How oxygen complicates iron uptake
Iron can be tricky to manage because it is very reactive. It exists in a variety of ionic states (with charges anywhere from +6 to -2) but exists primarily as Iron2+(II; Ferrous Iron) or Iron3+(III; Ferric Iron) and transitions readily between them depending on environmental variables like oxygen.
Unfortunately, because it is highly reactive, iron is typically unavailable.
It flits between soluble and insoluble forms, forms compounds with other minerals and (generally in aerobic environments) plays hard to get.
The problem? Ferrous iron is available to plants (it’s soluble). Ferric iron is not (it’s insoluble). Ferric iron is the more oxidized form, whereas ferrous iron is less oxidized.
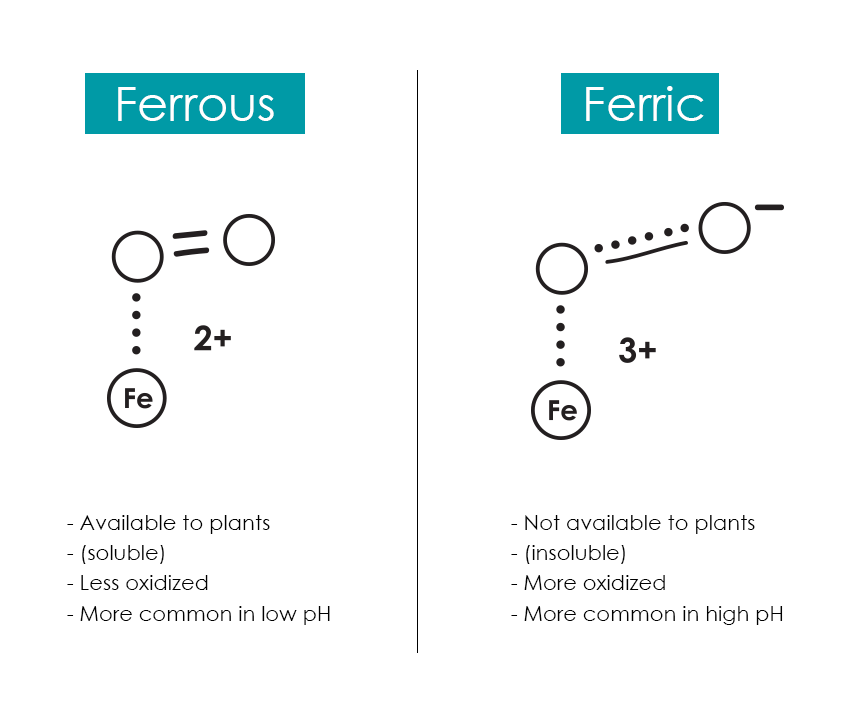
Things grow more complicated because as soon as ferrous iron becomes soluble in aerobic environments, it is often oxidized (becoming ferric iron) OR it reacts with other compounds to become biologically unavailable (especially at high pH values when different hydroxides are formed).
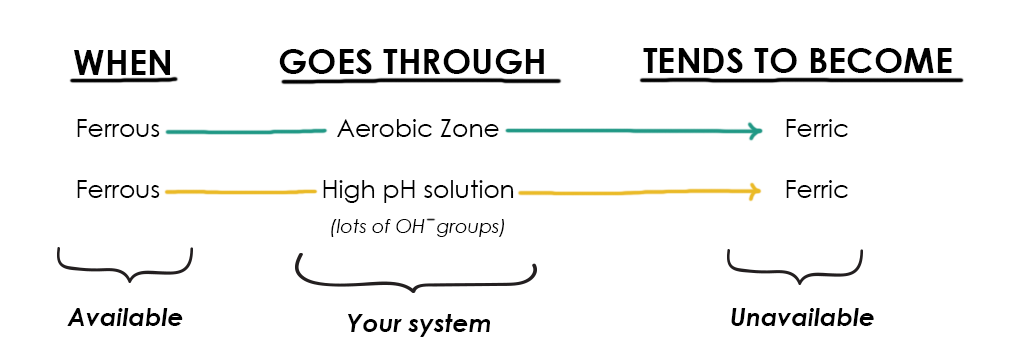
Because systems are generally aerobic (and certainly aerobic in the root zone), iron deficiencies can often arise—even when there is technically plenty of iron within the system.
Now, this relationship between oxygen and iron isn’t a full-time thing. In reality, iron is flitting between ferrous and ferric states, but the dominant state in high-pH and oxidized environments is ferric; this means that your plants cannot take it up.
These details are important because they dictate how we examine the solutions.
Supplementing iron in aquaponics—wrong approaches
Many practitioners throw rusty iron items into their systems falsely assuming that this will supplement system iron.
In a sense it does add to the reservoir of system iron, but not in a constructive or meaningful way. All this does is introduce more ferric iron to the system—a form of iron that was most likely already in plentiful supply.
Other practitioners intentionally develop dedicated anaerobic zones, where ferric iron will be reduced by the oxygen-free anaerobic environment to produce ferrous iron. This is a more compelling approach, especially in low pH systems, but still does not entirely address the problem of getting the reduced iron ion (Fe2+) through the oxygenated aerobic zone surrounding the plant roots (especially in high pH systems where hydroxyl ions are plentiful!).
In low-pH systems, ferrous iron has a much better chance of reaching the root zone, simply because there are fewer hydroxyl (OH–) groups to react with along the way. However, even in the absence of hydroxyl groups, there are many other chemical obstacles to reaching the plant root zone in adequate quantities.
The good news is that the problem of iron uptake is a problem that has not been overlooked by nature.
The plant’s solution: chelation
Plants have some amazing chemical means of hijacking ferric iron ions, tying them up, bundling them into the soluble, biochemical equivalent of the panel van, and delivering them, bound and gagged, to the root surface for plant use.
Plants also use a few other techniques to make iron available, including acidifying the root surface by excreting hydronium (H+) ions and secreting iron reducing compounds. But for the sake of aquaponic system management, this first biochemical iron-fixing technique is what we will focus on.
This process is called chelation—that is, tying insoluble ferric iron ions and compounds to organic molecules to make them soluble.
Chelation is accomplished by special organic molecules called chelatins or chelating agents. These are organic molecules that are specially designed to capture, or “dissolve” metals, of which iron is one.
In the plant world, chelatins are produced by the plant roots and leaked into the soil to capture and deliver insoluble iron ions.

The most effective of these chelating agents are phytosiderophores which bind iron ions very strongly, pulling them from the various insoluble precipitates and substances in which they most commonly occur. These chelating agents are special compounds produced by certain plants (phytosiderophores) and bacteria (siderophores) that are incredibly effective at binding iron. Grasses (Poaceae), and especially barley are particularly effective at producing phytosiderophores for capturing iron.
(As a side note: a great deal of research is being done on using barley to produce siderophores for iron sequestration, and holds some interesting implications for aquaponic systems where practitioners are willing to grow barley.)
Common chelating agents in your system
Other common chelating agents are amino acids, organic acids (especially humic acids), and polyphenols.
These are compounds that help keep the iron soluble and biologically available to the plants and algae in the system. While these compounds can be introduced, and humic or “tea-water” solutions can be fostered and managed, they aren’t always enough to keep iron available to the plants—especially in systems with a pH of 7 or above. In these systems, an artificial chelatin is often required.
The use of peat potting mixes (for seedling germination and transplants) allow growers to maintain high levels of humic substances. However, it’s still beneficial to supplement chelated iron regularly.
Iron is one of the plant nutrients that must be supplemented in almost all aquaponic systems.
How to supplement iron in aquaponics
To supplement iron, chelated iron must be added to systems.
Admissible under USDA Organic standards, chelated iron is an artificially chelated iron ion—essentially, iron attached to an organic molecule to make it soluble.
By adding chelated iron, iron deficiencies in your plants can be avoided.
Forms of chelated iron
The most common forms of chelated iron are:
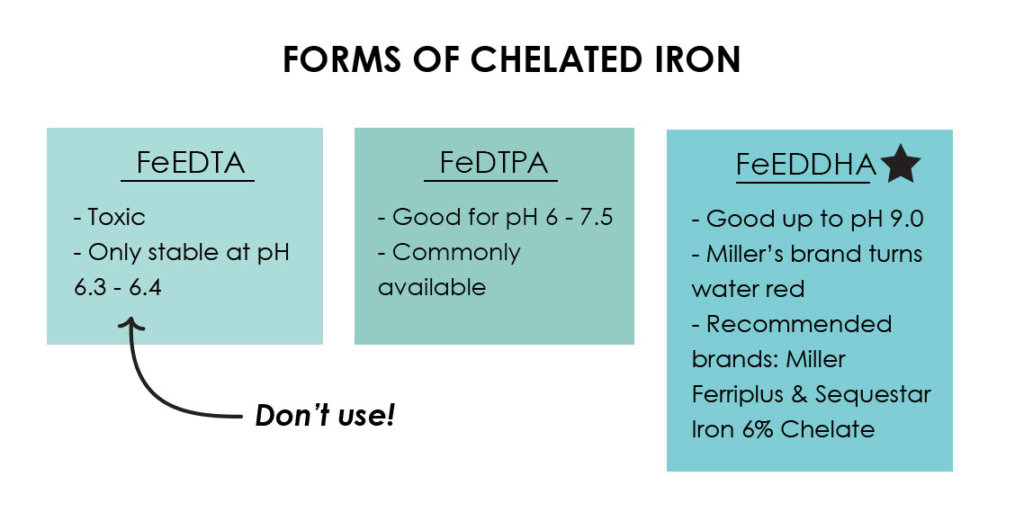
FeEDTA: This is a slightly toxic form that aquaponic practitioners should not use. This type of chelated iron is commonly used as an herbicide to kill broadleaf weeds. It should not be used just because of its toxicity, but also because it typically only effectively chelates iron up to the pH range of 6.3 or 6.4. Above this range, it is not a stable chelate. So, using FeEDTA in your consistently pH 7.0 system represents a significant amount of money wasted in comparison to other forms of chelated iron. For this reason, I recommend that AP practitioners do not use FeEDTA. It is ironic that this is the most commonly sold and used form of chelated iron in aquaponic systems as it is fairly ineffective—the equivalent of modern “aquaponic snake oil.”
FeDTPA: This is what I recommend for most systems at pH values between 6 and 7.5. It is commonly available at lawn and garden stores. (Get it online here.)
FeEDDHA: This is what I recommend for systems which have pH values up to 9.0 (let’s hope your pH never gets that high!), and the best all-around form of iron chelate, especially for starting systems. Effective at a broad pH range, FeEDDHA maintains iron solubility in almost all of the water conditions encountered by startup aquaponic systems
Chelated iron fertilizer is available from many different suppliers and usually easy to find at a local hardware store.
Common thinking about adding chelated iron
There are two schools of thought on chelated iron addition.
1) Addition in response to deficiency
Some say that chelated iron should be applied anytime you see a deficiency. This is a reasonable and reactionary dosing method but ultimately means that your plants must first suffer from iron depletion and deficiency before the problem is addressed. In this scenario plant production can be negatively impacted.
2) Addition on a regular basis 
The other (and better) school of thought is to apply iron at the standard UVI system rate of 2mg/L every three weeks. (Dr. Rakocy at the University of the Virgin Islands was the first to design a standard DWC aquaponic system. Many of our aquaponic numbers and ratios today are drawn from his research.) For more details on how much iron to use, watch Iron in Aquaponics – Part 2 (How much do I need?)
Iron can also be applied through a foliar application—using either chelated iron or ferrous sulfate mixed at low concentrations. Foliar application is great for fast response. But because iron isn’t a mobile nutrient inside plant tissues, iron will have to be supplemented regularly using this method—a time consuming, and ultimately less effective iron supplementation method.
In summary, iron can be regularly dosed so that iron deficiencies do not arise in your system.
Cost of Chelated Iron
While many practitioners complain about the cost, when bought in the 5–10-pound bag, chelated iron is really not very expensive, and often even in large commercial systems, will last for many months.
At the dosing rate above, a 10 pound, $15 bag of chelated FeDTPA will last well over a year or less than $1 per month. At higher iron concentrations it will last much longer.
Chelated iron products
We’ve had several folks ask about where they can get good iron supplements and how much they cost. Here is that info:
- “Miller DP”- DTPA (On the shelf or ordered through Ace hardware)
- “Sequestrene”- DTPA (5 lbs bag on Amazon for $57)
- “Miller FerriPlus”- EDDHA (SunshineGardensFl.com; 1 lb for $20, or 20 lbs for $300)
- “Sequestar Iron 6% Chelate” – EDDHA (RoseCare.com; 5 lbs for $73)
All of these products will work great in your system!
*Quick tip: red dye in Miller’s FeEDDHA!
A friend and blog reader let us know about this product that seemed to have turned his entire system water red after adding 3 ounces. It appears the Miller’s product contains red dye! A dye probably won’t hurt your fish, but double check to be sure.
We hope that this blog post is helping you keep your system healthy. To learn more about aquaponic systems, check out our Youtube channel.
Thinking about starting an aquaponics business?
Upstart University is the gateway that brings aquaponicists from hobby mode to business mode. Starting a business involves many principles (like business planning, financial analysis, finding markets and choosing a sales approach) that are often unfamiliar to new growers.
Want to navigate the entrepreneur journey quickly and efficiently? Check out the courses at Upstart University. You can get a free trial to try out the platform today.




Wow! What a great overview of iron uptake in plants! We will definitely share this with our student interns and customers!
We definitely tried an iron supplement with the red dye- it turned our system red for a week and stained our white pond liner pink!!! Whoops! At least it did not harm our fish!
Now we use AquaIron for all of our aquaponics needs.
question, running a UV sterilizer to keep the green water down in an aquaponic system. There seems to be evidence the UV will make the iron convert to a form that is not available?
Hi Mark,
Don’t add iron while the UV is running. This will create precipitates, which means that the nutrient is unavailable to plants. Turn off the UV while supplementing with chelated iron, and then turn it back on once the system has cycled.
Mark, UV lighting causes the iron to precipitate out the water becoming unavailable to the plants. Try run you system without the UV lights for 24 hrs to 36 hrs a week and at the same time do a system adjustment with some iron.
Wow! What a great overview of iron uptake in plants! We will definitely share this with our student interns and customers!
I ma looking into “Certified Organic” for an aquaponics farm. Synthetic chelating agents are explicitly prohibited. What are some non-synthetic products for introducing iron into an aquaponics system?
Hi Christopher! We recommend this: http://www.safergro.com/products/biomin-iron/
Hello. I have a qestion. In the end what range of Fe values we need to have?
Hi Anastasia,
That will depend on your system. Because each system is unique, it is difficult for us to recommend exact ranges for nutrient concentrations—just make sure that you don’t see any deficiencies, and if you do, dose slowly so you don’t over correct and throw the system further off. The more you do it, the better feel you’ll get for the acceptable ranges of iron in your system.
There seems to be evidence the UV will make the iron convert to a form that is not available?
Hi David,
Yes, UV does cause iron to precipitate out of a solution. If this is a problem you’re experiencing, try unplugging the UV for 24–48 hours after dosing iron.
Excellent and perfect thanks for you
Excellent and perfect
This is very viable information. Can we get iron this from fish wastes.
Hi James,
Iron is not abundant in fish waste. In fact, most aquaponic systems require iron supplementation (chelated iron).
Nice post, thanks for sharing!
A very enlightening post, however Sequestar Iron 6% Chelate also turned my water red.
How long does the red color stay? Is it until enough water is added to dilute the color out or does it get consumed eventually by the plants?
Also, I had a very hard time finding a 10lb bag of chelated iron for $15—can you please link to some websites? My system is quite large, and the cost of iron is significant.
Thanks in advance!
Very comprehensive and complete
Just FYI sequestar Iron turns your water red. Also, I’m not able to find iron at the prices listed in the article—can anyone recommend where to buy iron at 10lb/$15?
Can you share your source for the above statement:
“Admissible under USDA Organic standards, chelated iron is an artificially chelated iron ion—essentially, iron attached to an organic molecule to make it soluble.”
I was under the impression, synthetic chelates are not allowed
What about Iron gluconate? I’ve found it to work immediately and best Iron for the buck
Do you have a site or listing to show where chelated iron is “Admissible under USDA Organic standards” I’ve searched omri.org and only see one brand – biomin.
I don’t have access to biomin and we are using a FeEDDHA – 6% water soluble, 3% chelated with EDDHA, and 2% Chelated with EDDHA. We are not pursuing Organic, but get ask if we are using organic fertilizers. We use an Italian brand and it does say on the label – “allowed for biological agriculture” under EU legislation.
Any help on U.S. information or listing for our confidence and general knowledge will be helpful Thank you!
Thank you!
Can you address evaporation? Do I need to account for iron loss during evaporation, or only iron lost due to water changes/removal?
Hey, my local nursery only had a Fertilome Chelated Liquid Iron and Other Micronutrients. It doesn’t say anywhere on the label…or on their website which kind of Fe it is. Are you familiar with this product? Is if safe for my aquaponics system and my 80 baby trouts?
I’m from Pakistan I have seen your blog, I appreciate your work you are a role model for new entrepreneurs. I’m doing aquaponics in Pakistan and I need your little bit of support regarding how to meet iron criteria using organic or artificial? How many doses are required for a 3000 sqt aquaponics system that’ll not affect our system? How to produce organically?