pH in Hydroponics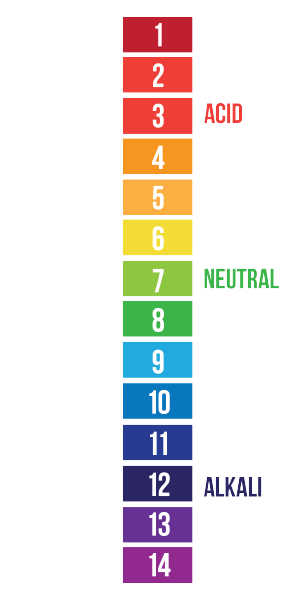
When it comes to learning about hydroponics and aquaponics, it’s likely that you’ll run into a few unfamiliar terms. In particular, there’s a lot of mumbo-jumbo about pH—we know it’s important and we know that it affects the way our plants grow in the system. But what exactly does that mean; why does it have such a big impact on the success of our farms? Let’s dig into the chemistry a bit.
pH is a measure of acidity, which matters because nutrients are more or less available at different levels of acidity. That means you’ll need to find the sweet spot on the pH scale where all of the nutrients are available, and the plants are getting what they need.
pH is represented by a scale that ranges from 0 to 14 (0 being the most acidic and 14 being the most basic). The term “pH” stands for “potential hydrogen” because hydrogen ions (H+) and hydroxide ions (OH-) are what cause a solution to be basic or acidic. pH tests measure the concentration of these two substances in a solution. Because there are so many, measuring a number of hydroxide or hydrogen ions is not practical, so instead, pH is measured on a logarithmic scale (we’ll get to that shortly).
This is where things get more complicated. As you know, water is made up of hydrogen and oxygen. Two hydrogens, in fact. Pure water has a pH of 7, which means that it has an equal number of hydrogen and hydroxide ions because nothing else is in the water to break up the atoms.
As water molecules break up, (which happens when a nutrient mix reacts with water), the water becomes acidic or basic. Another way of saying this is that water molecules are broken down and hydrogen and hydroxide ions are formed. pH depends on which is formed in higher concentration. We measure the concentration of hydrogen in relation to hydroxide; the critical measure is the hydrogen ion concentration.
Looking at the numbers
Some solutions contain more hydrogen ions than hydroxide ions, therefore making them acidic. These solutions would have a pH value of 0–6.9. Others contain more hydroxide ions, which makes them basic, so they would have a pH value of 7.1–14. If a solution has an equal concentration of hydronium and hydroxide ions, it is neutral (pH 7). Let’s take a closer look at those numbers.
pH numbers represent a logarithmic scale. So, if your pH meter says 6.5, that doesn’t mean that there are 6.5 hydrogen ions in your system. It means that the concentration of hydrogen ions is five times higher than a neutral solution (pH 7). An acidic solution can have a hundred trillion times more hydrogen ions than a basic solution, which can be quite cumbersome to represent numerically. This is why scientists use the pH scale. Each 1 full unit change in a pH number represents a tenfold difference in the actual concentration. So a pH of 0 has a hydrogen ion concentration that is ten times higher than a pH of 1, which has a concentration that is ten times higher than a pH of 2, and so on.
What does this mean for your system?
It’s important to understand how acidic or basic your solution is because it tells you a lot about nutrient availability, the hardness of your source water, and it can help you identify deficiencies. pH is also one of the reasons that a lot of people choose to use a reverse osmosis filter, which can remove mineral content and thus the buffering capacity (we’ll get to that) of the water.
For the most part, nutrients are most available between a pH range of about 5.8–6.5. What does that mean? Why do we keep saying this? Some forms of nutrient are unavailable to plants, which means that even if you add it to your system, your plants can’t use it. You want to make sure that the pH in your system is within the right range. Let’s take iron for example:
Iron comes in two forms—ferric and ferrous. Ferric has a charge of +3 and is unavailable to plants. Ferrous iron, on the other hand, has a charge of +2 and is available to plants. Iron fluctuates between these two forms in your system. As it is, ferrous iron can quickly become ferric iron in a system with a high pH because of the way it reacts with hydrogen ions. Therefore, in higher pH ranges, plants can quickly develop iron deficiencies. To learn more about the way that iron interacts with other elements in the system, read the Iron in Aquaponics blog post.

Inversely, adding nutrients to your system can affect the pH. For the most part, hydroponic nutrients will drive pH down (or cause the solution to become more acidic). Some nutrients will react with the hydrogen and hydroxide ion concentrations, and some contribute hydrogen or hydroxide to the system.
You can also influence pH by adding pH down (an acid) or pH up (a base). pH down is primarily phosphoric acid, which is the most stable and least harmful to your plants. It is also safe for fish, which means you could use it in an aquaponic system.
Fun fact: if you use ammoniacal nitrogen—instead of nitrate—in higher levels, it causes your root zone to release more protons, which will drive the pH down.
Buffering capacity
Buffering capacity is the ability of a solution to resist a change in pH. Where that most commonly comes into effect in hydroponics or aquaponics, is that most people’s source water is hard water, which has a lot of carbonates. These are extremely good at buffering the water at a high pH. So if you consistently have high pH in your system, and adding pH down doesn’t help, it’s likely because the carbonates in your solution are increasing its buffering capacity. Have your source water tested, and if you see a high level of carbonate, you’ll probably want to use an RO filter to sort out those minerals.
Here are some familiar things with acidity that will help you orient yourself to pH:
|
|
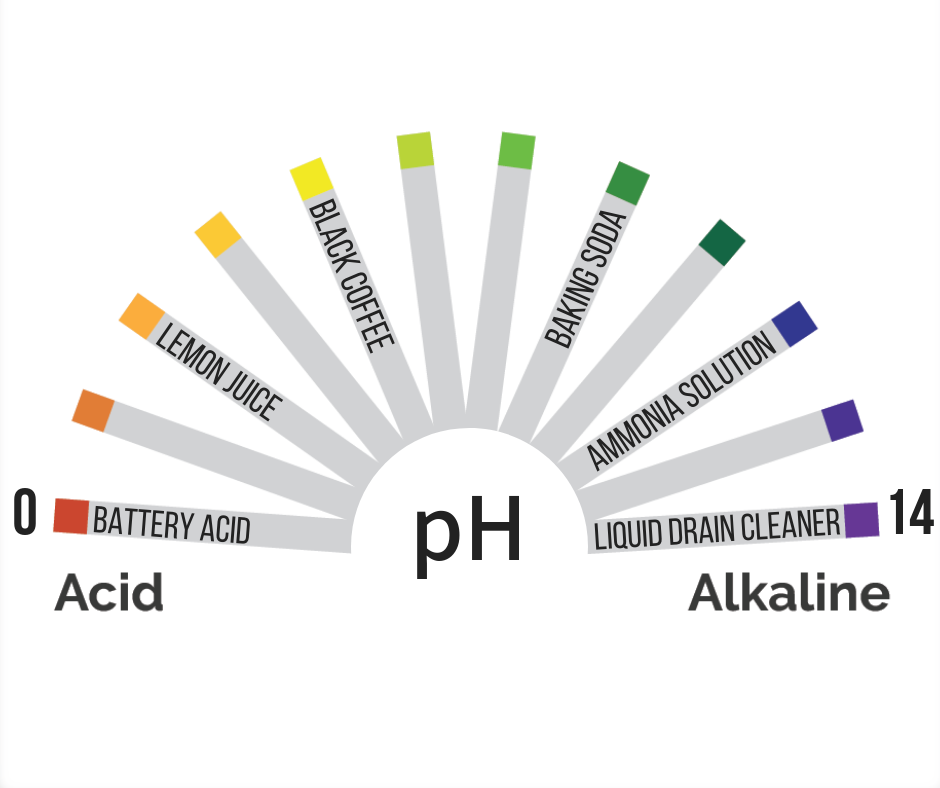
This doesn’t mean you should go putting these items into your system to alter your pH levels. In fact, that would be a terrible idea. Remember that acidity in your solution needs to be fairly stable because it affects how available nutrients are to the plants. Take a look at the pH chart to determine the acidity at which the plants in your system can use the nutrients you provide them. Be strategic about dosing your system and remember to account for the buffering capacity. Use approved chemicals like pH up or pH down if you need to alter the levels. Just like everything else, practice makes perfect, so the more exposure you have to your system, the better prepared you’ll be to stabilize pH levels!
Learn more about pH in hydroponic and aquaponic systems by taking these Upstart courses:
reading these blogs:
and watching these videos:



We mainly use nitric acid in Tank Z to reduce pH between 5.5-6.5 for melons and strawberries grown in coco coir.We are distributing locally both organic fertilizers ( containing fulvic acid and humic acid) and chemical ones which depend on the seasons and the cultivars.We are selling a lot of potassium phosphite on which I would like to have your comments.Actually I don’t use it much in my GH.Thanks.
My measurement shows – – – – Can you tell me what this means, please?
(I am new to the hydroponic system)